Hydrogen storage is very challenging. Hydrogen can be stored using various technological methods, for example in a form of metal hydrides. Metal hydrides are chemical compounds in which hydrogen is bonded chemically to a metal or metalloid element. Metal hydrides can be powders or liquids that are usually stored in tanks at a pressure of ~1-3 MPa. Examples of metal hydrides are MgH2, NaAlH4, LiAlH4, LiH, LaNi5H6, TiFeH2, palladium hydride, ammonia borane, etc.
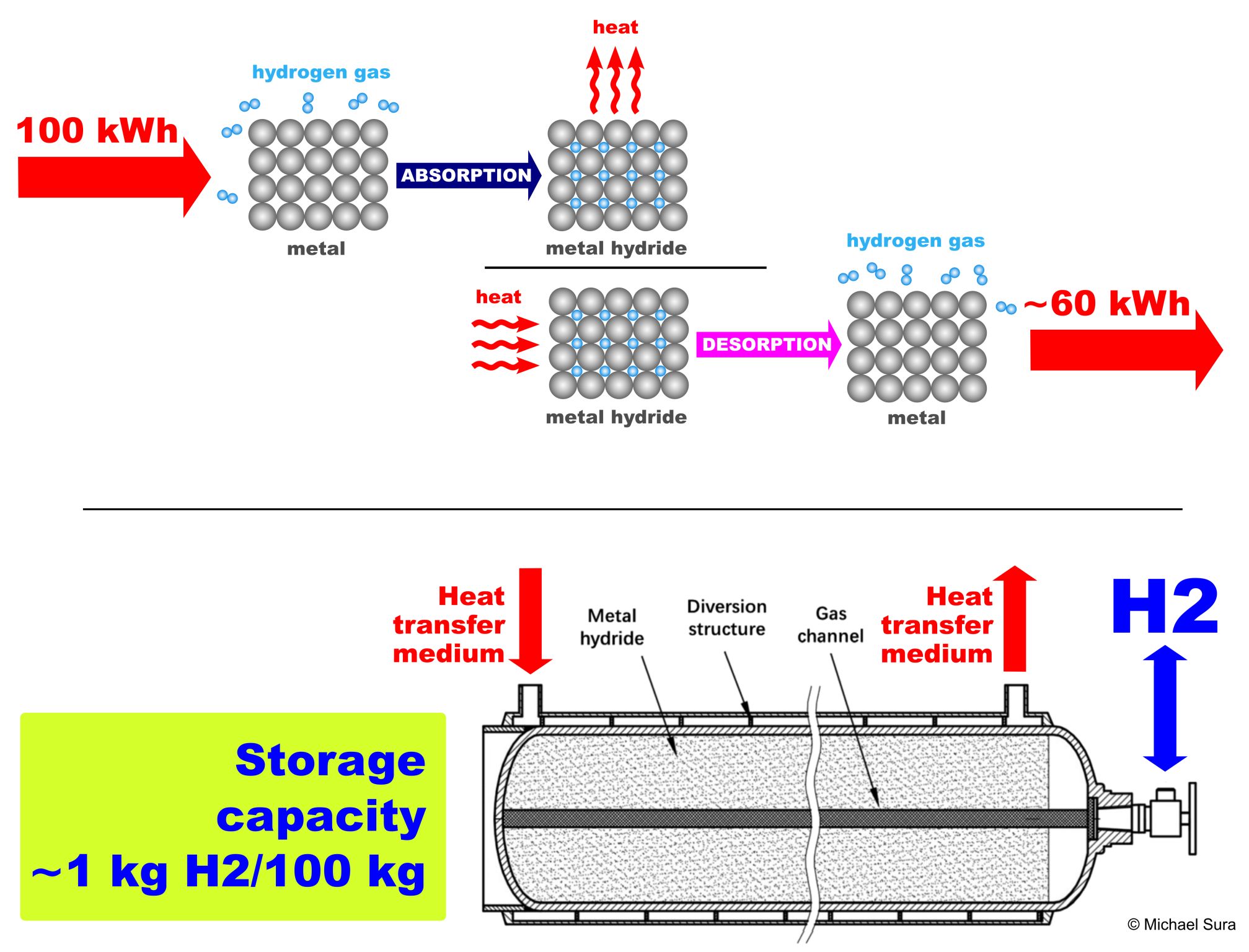
The principle of operation of the metal hydride tank for reversible hydrogen storage is following. Hydrogen is stored by reacting with a metal to form a hydride (exothermic reaction) at a pressure of 1-3 MPa and is released from the hydride (endothermic reaction) by heat (~120-200 °C).
Proponents of hydrogen consider metal hydrides as very safe materials for solid-state hydrogen storage under mild conditions and with high volumetric densities.
But the storage capacity of the metal hydride storage tank is ~ 1kg H2/100kg of metal hydride material. Metal hydride pressure tanks are very heavy!
Storing hydrogen in metal hydrides is very energy intensive and only ~60% efficient!
There exist several projects where buses or passenger cars where is intended to power them using fuel cells supplied with hydrogen from metal hydride tanks (financed mostly from public funds). What a waste of money, energy, and so on.
It is another proof evidence that hydrogen, when used for energy purposes, is a fuel of despair.






