Kỹ thuật vi sóng mới có thể biến CO₂ thành nhiên liệu hiệu quả hơn nhiều
Đại học Tokyo, 19 tháng 11, 2025
Một phương pháp gia nhiệt dựa trên vi sóng đột phá do các nhà nghiên cứu tại Đại học Tokyo phát triển có thể cải thiện đáng kể hiệu quả của các phản ứng hóa học, mở ra khả năng sản xuất nhiên liệu xanh hơn và tái sử dụng carbon dioxide (CO₂).

Gia nhiệt chính xác cho hóa chất công nghiệp
Nhiều quy trình sản xuất hóa chất công nghiệp dựa vào nhiệt, nhưng các phương pháp gia nhiệt truyền thống thường lãng phí năng lượng khi làm nóng những khu vực không tham gia trực tiếp vào phản ứng. Nhóm nghiên cứu, do giảng viên Fuminao Kishimoto thuộc Khoa Kỹ thuật Hệ thống Hóa học dẫn đầu, đã phát triển hệ thống tập trung năng lượng chỉ vào nơi cần thiết.
Phương pháp này sử dụng vi sóng—tương tự như lò vi sóng gia dụng—để kích thích chọn lọc các nguyên tố cụ thể trong vật liệu phản ứng. Hệ thống này đã đạt hiệu suất năng lượng cao hơn khoảng 4,5 lần so với phương pháp truyền thống.
“Trong hầu hết các phản ứng hóa học, chỉ có những vùng rất nhỏ liên quan đến một vài nguyên tử hoặc phân tử thực sự cần năng lượng,” Kishimoto giải thích. “Gia nhiệt truyền thống phân tán năng lượng khắp lò phản ứng, rất lãng phí. Chúng tôi nhận thấy vi sóng có thể tập trung năng lượng vào các vị trí nguyên tử đơn lẻ, giống như cách lò vi sóng làm nóng thực phẩm.”

Vật liệu này trông giống như một tảng đá phủ băng, nhưng dưới kính hiển vi, bạn sẽ thấy một mạng lưới giống như bọt biển. Đây là chìa khóa cho các thí nghiệm vì các khoang có thể được lấp đầy bằng các ion cụ thể để tạo ra nhiệt từ vi sóng. Vì vật liệu này xốp, chất lỏng có thể chảy qua, hấp thụ nhiệt để kích hoạt các phản ứng. Nguồn: 2025 Hannes Grobe CC-BY-SA-2.5
Nguyên lý hoạt động
Không giống lò vi sóng thông thường nhắm vào các phân tử nước ở tần số 2,45 gigahertz, các nhà nghiên cứu điều chỉnh vi sóng ở tần số 900 megahertz. Tần số này tối ưu cho việc kích thích zeolite—vật liệu xốp có khả năng hấp thụ và truyền nhiệt hiệu quả.
Bên trong các khoang zeolite, các ion indium hoạt động như ăng-ten hấp thụ năng lượng vi sóng và sinh nhiệt. Nhiệt sau đó được truyền đến các hóa chất đi qua cấu trúc xốp, giúp phản ứng diễn ra tại các vùng cục bộ với hiệu quả cao. Để đạt được độ chính xác này, nhóm nghiên cứu đã phát triển trong bốn năm tại cơ sở bức xạ synchrotron lớn của Nhật Bản, SPring-8.
Ứng dụng trong sản xuất nhiên liệu và tái chế carbon
Nhờ khả năng tập trung nhiệt chọn lọc, các phản ứng thường đòi hỏi nhiệt độ cao, như phân tách nước hoặc chuyển hóa methane, có thể diễn ra ở điều kiện nhẹ hơn. Kích thước lỗ xốp của zeolite có thể điều chỉnh để tối ưu hóa hiệu suất và tính chọn lọc của phản ứng.
Kỹ thuật này cũng có tiềm năng trong thu giữ carbon, chuyển hóa CO₂ thành hóa chất hữu ích và tái chế nhựa hiệu quả hơn.
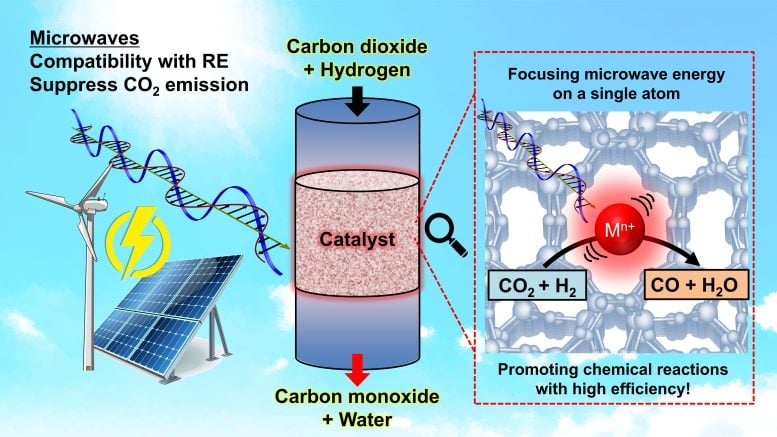
Lý tưởng nhất là các phản ứng vi sóng có thể được thúc đẩy bằng năng lượng xanh, trong trường hợp đó, hệ thống có thể giúp giảm carbon dioxide bằng cách chuyển đổi nó thành các hóa chất hữu ích khác. Nguồn: 2025 Kishimoto và cộng sự. CC-BY-ND
Thách thức phía trước
Việc mở rộng phương pháp từ thí nghiệm phòng thí nghiệm sang sản xuất công nghiệp vẫn là thách thức. Vật liệu sử dụng phức tạp, đắt tiền, việc đo nhiệt độ ở quy mô nguyên tử khó khăn, và cần cải tiến thêm để giảm tổn thất nhiệt và điện.
“Chúng tôi hướng tới việc mở rộng ý tưởng này sang các phản ứng hóa học quan trọng khác và tối ưu hóa chất xúc tác để tăng độ bền và khả năng mở rộng,” Kishimoto nói. “Các thí nghiệm quy mô thử nghiệm có thể diễn ra trong thập kỷ tới, với việc áp dụng rộng rãi phụ thuộc vào tiến bộ công nghệ và hạ tầng năng lượng. Chúng tôi đang tìm đối tác doanh nghiệp để phát triển chung.”
Nguồn: Ishibashi, R., Kishimoto, F., Yoshioka, T., Yamada, H., Muraoka, K., Ina, T., Taniguchi, H., Nakayama, A., Wakihara, T., & Takanabe, K. (2025). Focused thermal energy at atomic microwave antenna sites for ecocatalysis. Science Advances.






