Scientists Develop the World’s First Rechargeable Hydride Ion Battery
By Dalian Institute of Chemical Physics, Chinese Academy of Sciences – October 1, 2025
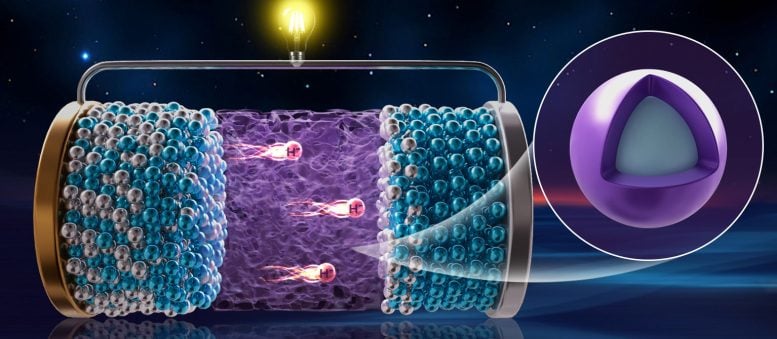
Researchers at the Dalian Institute of Chemical Physics (DICP), Chinese Academy of Sciences, have developed the first rechargeable hydride ion (H⁻) battery. Hydride ions are promising charge carriers due to their very low mass and high redox potential, but progress had been limited by the lack of suitable electrolytes.
The team designed a core–shell hydride ion electrolyte called 3CeH3@BaH2, where a thin BaH2 shell encapsulates CeH3. This structure combines the high ion conductivity of CeH3 with the thermal and electrochemical stability of BaH2, enabling fast hydride ion movement at room temperature.
They assembled a CeH2|3CeH3@BaH2|NaAlH4 all-solid-state prototype battery. Using NaAlH4 as the cathode, the battery delivered an initial discharge capacity of 984 mAh/g at room temperature and retained 402 mAh/g after 20 cycles. In a stacked configuration, the battery achieved 1.9 V, enough to power a yellow LED lamp.
By using hydrogen as the charge carrier, this technology avoids dendrite formation, offering a safe, efficient, and sustainable energy storage solution. Hydride ion batteries could play a major role in clean energy storage and conversion.
Reference:
Cui, J., Zou, R., Zhang, W., et al. “A room temperature rechargeable all-solid-state hydride ion battery.” Nature, 17 September 2025. DOI: 10.1038/s41586-025-09561-3






